Packaging reliability of lithium-ion batteries is very important for the safety performance of batteries. If packaging problems occur, battery swelling and leakage will be caused, which seriously affects the performance and safety of batteries. Shell voltage is the voltage between the positive ear and the aluminum layer of the aluminum-plastic film, and its value can represent the encapsulation effect of the aluminum-plastic case.
Shell voltage is divided into two types: the voltage between positive ear and shell, and the voltage between negative ear and shell. When the voltage of negative shell is large, the battery shell will be charged, and fire will occur in the process of use, but it will not corrode the shell. When the voltage of the positive lug shell is relatively large, the corrosion of the aluminum plastic shell will cause the swelling and leakage phenomenon of the battery. When the positive shell voltage is high, it indicates that the aluminum-plastic film PP layer has been damaged, and the aluminum layer has been in contact with the electrohydraulic in the battery.
When the shell voltage is greater than 1.0V, reaching the formation voltage of lithium aluminum alloy, and the electronic path at the negative ear also exists, the electrohydraulic lithium ions will be embedded in aluminum, forming lithium aluminum alloy, resulting in corrosion of the aluminum shell. As the storage time increases, after the corrosion of the aluminum layer, the external moisture enters the battery, reacts with the electrolyte and active substances, produces gas, and finally the battery bulges and leaks.
The risk of shell corrosion can be identified by the test of shell voltage, but how to reduce the voltage of battery shell and ensure the stability of battery shell voltage in mass production is often encountered in the production process. In this paper, through the study of the rule of shell voltage, starting from the mechanism of shell voltage generation to improve the shell voltage level, using the DOE research method, effectively improve the shell voltage level.
1.1 Shell voltage test method
Shell voltage is to test the voltage between positive lug or negative lug and aluminum plastic film. This paper mainly studies the voltage between positive lug and aluminum plastic shell. Set a MULTIMETER (UNI-T UT56 MULTIMETER) or Agilent (34401A) to DC voltage and check that the meter is normal. Then, touch the positive electrode of the battery with a red pen, and touch the aluminum foil in the middle layer at the edge of the shell with a black pen and slip slightly. The pen should fully contact the aluminum layer in the aluminum-plastic packing bag.
1.2 Copper plating analysis
Exhaust electrical core cutting front take out the pole group, keep the aluminum-plastic bag; Then, 10% copper sulfate solution is poured into the aluminum-plastic bag, and 3.7V DC power supply is connected externally. The negative probe penetrates the aluminum-plastic bag to make the negative electrode and the aluminum layer of the aluminum-plastic bag through. The aluminum-plastic bag contains the copper sulfate solution, and the positive probe is immersed in the solution, and the solution is poured out after 20min test to observe the copper dissolution inside the aluminum-plastic bag. If the PP layer is damaged, the copper sulfate solution will contact the aluminum layer directly and reduce to copper on the surface of the aluminum layer.
1.3 High-power microscope test analysis
After the experiment, the encapsulation of the aluminum-plastic shell was taken, and the position to be observed was dissected with a cutting machine (REM-710, made in Japan), and the profile was observed under a high-power microscope of VHS(VHX-6000, made in Shanghai, China) to observe the sol morphology of PP layer, the absence of PP layer and the position of copper analysis.
1.4 Experimental scheme
Two groups of soft pack polymer battery 616072, model 476384, and 24 batteries in each group were selected. Multimeter and Agilent meter were used to test the shell voltage, and video was used to record the whole reading change, and then all displayed values were read in turn in slow play to study the shell voltage change rule. Multimeter is used to test the shell voltage of batteries with different flow and different folding modes, and study the variation of shell voltage with time and folding mode. Use a multimeter to select batteries with shell voltage < 0.8V, 0.8~1.0V, 1.0~1.2V, 1.2~2.0V, 2.0~3.0V, > 3.0V for electroplating analysis of its risk, cutting analysis of copper analysis position, study its causes, analysis of improvement methods.
2.1 Variation rule of shell voltage
FIG.1 shows the voltage comparison between the battery case 476384 tested by a multimeter and an Agilent meter. The first number is the largest, then it drops rapidly, and then it flattens out from the third number. Agilent test results are significantly larger than multimeters.
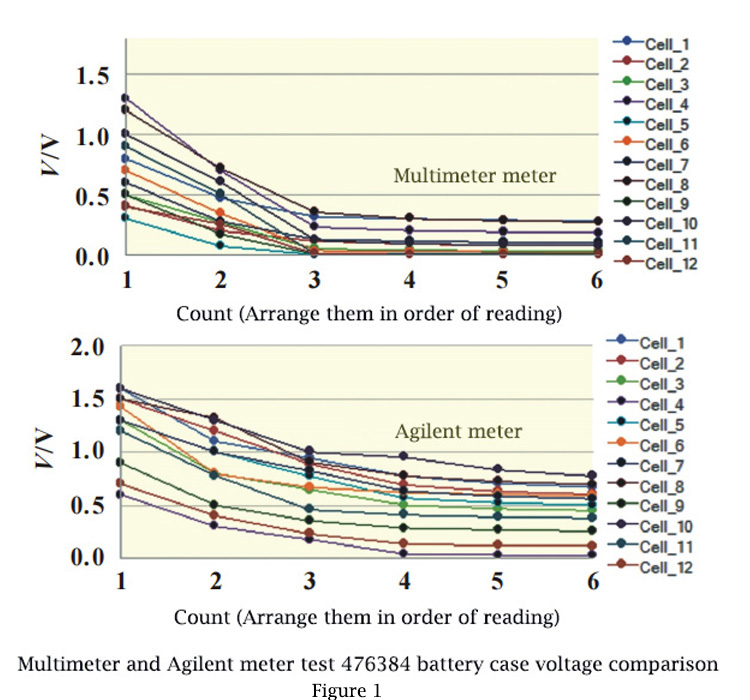
Theoretically, the aluminum layer between the positive electrode or the negative electrode and the aluminum plastic film is insulated, and the voltage of the shell should be 0V; However, in the actual process, the aluminum-plastic film will be locally damaged, resulting in local conduction (ion channel and electronic channel) between the positive and negative electrodes and the aluminum-plastic film, forming a micro cell, and thus a potential difference (voltage).
Ion channel: In the process of cell packaging, the PP layer at the edge sealing position is prone to damage after hot pressing. In addition, the folding process is also prone to damage the PP layer.
Electronic channel: aluminum-plastic film aluminum layer and nickel pole lug or anode contact.
At the beginning of contact, the concentration of ion is the highest and the polarization is also the highest, so the test value is the highest. The test value of shell voltage decreases rapidly and tends to be stable as ion diffusion tends to equilibrium gradually.
2.2 Repeatability of shell voltage
Twelve 616072 cells were randomly selected to measure and record the voltage of the case using a multimeter. The voltage was monitored continuously for 4 days to investigate the repeatability of the test value (FIG.2).
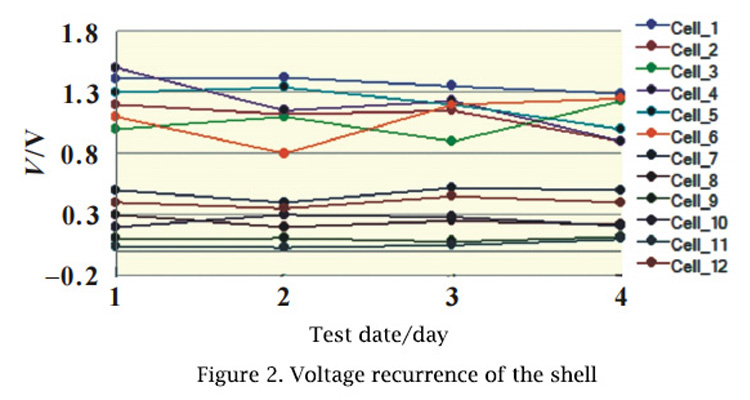
The sample results show that the shell voltage has poor reproducibility. Because the shell voltage is generated because the aluminum layer exposed at the damaged position of PP layer and the positive/negative ear form ion channels through the electrolyte, the result of each test is closely related to the concentration of ions. As well as the degree of folding, test time and test contact mode, the ion concentration may be inconsistent, so there are small differences in the results of each test.
2.3 Plating analysis of different shell voltages
3 batteries with different gradient shell voltages of model 616072 were selected with a multimeter and disassembled for copper plating experiment. The experimental findings: For the samples with shell voltage < 0.8V, there was no copper evolution in the electroplating experiment, indicating that the package with shell voltage < 0.8V was relatively reliable. For the three samples with shell voltage between 0.8V and 1.0V, spot copper evolution gradually appeared, indicating that the PP layer was slightly damaged at this time, and intensive and continuous copper evolution occurred in the batteries with shell voltage between 1.0V and 2.0V. This indicates that the risk of shell corrosion is relatively high.
No 2.0V ~3.0V shell voltage was found in production. No copper evolution was found in batteries with shell voltage > 3V. In this case, it is inferred that the electronic channel has been connected, that is, the negative ear is not well packaged, resulting in relatively high shell voltage. The experiment shows that the battery with shell voltage less than 0.8V has the lowest risk of shell corrosion, and the battery with shell voltage between 0.8 and 2V has the highest risk of corrosion as the shell voltage increases. When the shell voltage is greater than 3.0V, the electronic channel is connected and the corrosion risk is the highest.
2.4 Rule of variation of shell voltage over time
Two groups of 616072 batteries were randomly selected in the same batch and same process to design two sets of experiments: Group 1: Cell samples were extracted and folded first, and then the shell voltage value was tested before being put back into the original batch. Then, samples of this group were extracted to test the shell voltage value in each process and recorded; Group 2, referring to the sampling procedure of group 1, each procedure extracts the same amount of samples from the same batch of cells, first folds the edges, then tests the voltage value of the shell and records it.
According to the experimental method of group 2, the shell voltage value is gradually increasing. According to the experimental method of group one, the voltage value of the cell shell decreases gradually with the increase of time. According to the experimental results, there are two possible theories for the mechanism: Inference 1: After Degas, the internal electro-hydraulic permeability is not balanced, and the shell current is low. After enough standing time, the electro-hydraulic permeability reaches the equilibrium, and the shell voltage increases. Correlativity 2: After Degas, the PP layer is not completely cooled at the edge sealing, which has good tensile property and is not easy to be damaged, and the shell current is low. After standing, the PP layer is completely cooled and the tensile property becomes worse, and the PP layer is prone to damage after flanging, so the shell voltage is high.
In order to verify the above two inferences, the following experiments were carried out: After taking 24 cells with Degas, the edge was immediately folded to test and record the voltage value of the shell, and then the changes of the voltage value of the shell were continuously monitored and the results were observed. It is found that there is no significant change in the shell voltage with the increase of the standing time, and there is no increasing trend. Therefore, it is believed that the mechanism of Corollary 2 conforms to the experimental law.
2.5 Shell voltage generation principle and corrosion mechanism of battery
Step by step cutting the copper analysis position of the aluminum-plastic bag, it can be found that the copper particles are almost close to the aluminum layer (4mm away), as shown in Figure 3, but no significant damage point of PP layer can be found. It can be inferred that the damage of PP layer is non-macroscopic, but the formation of microchannels in the PP layer, connecting the free electrohydraulic and aluminum layer.
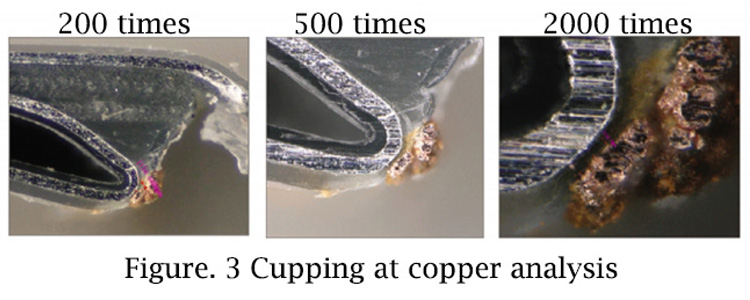
The PP layer morphology at the packaging position before and after folding is significantly different. It can be found that the PP layer at the crease position has a whitening phenomenon, while the cell with qualified shell voltage does not have this phenomenon. This phenomenon indicates that the mechanical properties of the PP layer after packaging have changed.
Damage of PP layer will lead to electrohydraulic contact with aluminum layer. When the voltage is greater than the formation voltage of lithium aluminum alloy, and the electronic path at the pole ear also exists, a tiny electrolytic cell will be formed. Lithium ions in the electrolyte will be embedded in the aluminum layer to form lithium aluminum alloy. The formation of lithium aluminum alloy leads to the destruction of the aluminum lattice, so that the aluminum layer is damaged, serious aluminum layer will be corroded through, nylon layer can not block water molecules, resulting in external water and electrolyte reaction, produce hydrofluoric acid; Hydrofluoric acid intensifies the erosion of the aluminum layer, resulting in the extension of the damaged area of the aluminum layer.
2.6 Shell voltages of different PP layers
FIG. 4 The shell voltages of 91, 113 and 158mm aluminum-plastic shells are used respectively. A single sealing machine was used to carry out encapsulation successively. After encapsulation, the test results of the shell voltage are as follows: blue is 91mm aluminum plastic, yellow is 113mm aluminum plastic, and green is 158mm aluminum plastic.
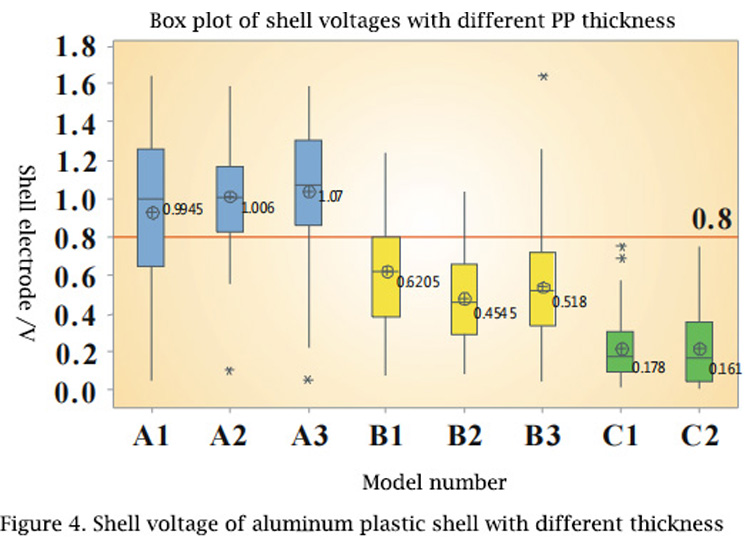
The thicker the aluminum-plastic shell is, the thicker the PP layer is. For aluminum-plastic shell with different PP layer thickness, the thicker the PP layer is, the lower the shell voltage will be. When the battery packaging parameters are the same, the PP layer that can be dissolved is the same, but for the aluminum-plastic shell with thick PP layer, it only damages or changes the surface of the PP layer, and the PP performance of the deeper part does not change, which can play a normal role of isolating electrolyte, so the shell voltage is low.
2.7 Improvement of Shell voltage According to the above mechanism, we can improve the shell voltage in two ways:
(1) The shell voltage can be improved by improving the performance of PP layer, improving its tensile property or increasing its thickness;
(2) The shell voltage can be improved by optimizing the process parameters and reducing the stretch of PP layer. Through the verification in production, the experimental effect is obvious, the shell voltage can be improved significantly.
This study shows that:
(1) In the continuous test of the shell voltage, the first value is the largest, and the latter gradually tends to be stable, and has a greater relationship with the reading accuracy of the measuring equipment, the shorter the reading interval of the equipment, the greater the measurement result;
(2) The reproducibility of the shell voltage is relatively poor, and the test results of the same battery are quite different;
(3) The battery with shell voltage less than 0.8V has no risk of swelling and leakage. Before 0.8~2.0V, the risk of swelling and leakage gradually increases with the increase of shell voltage, which is caused by ion pathway, and the battery with shell voltage greater than 3.0V is caused by electronic pathway;
(4) The mechanism of shell voltage generation is that the mechanical properties of PP layer after packaging are reduced, and the tension during folding causes the PP layer to generate tiny channels, forming an ion path between electrolyte and aluminum layer and generating shell voltage. The battery case voltage can be improved obviously by optimizing the process parameters, increasing the thickness of PP layer and changing the performance of PP layer.
Contact: Jason Wang
Phone: 13580725992
E-mail: sales@aooser.com
Whatsapp:13580725992
Add: No.429 Guangming Road, Shenzhen City, Guangdong Province
We chat
